Credits: University of Stuttgart / SFB 1313
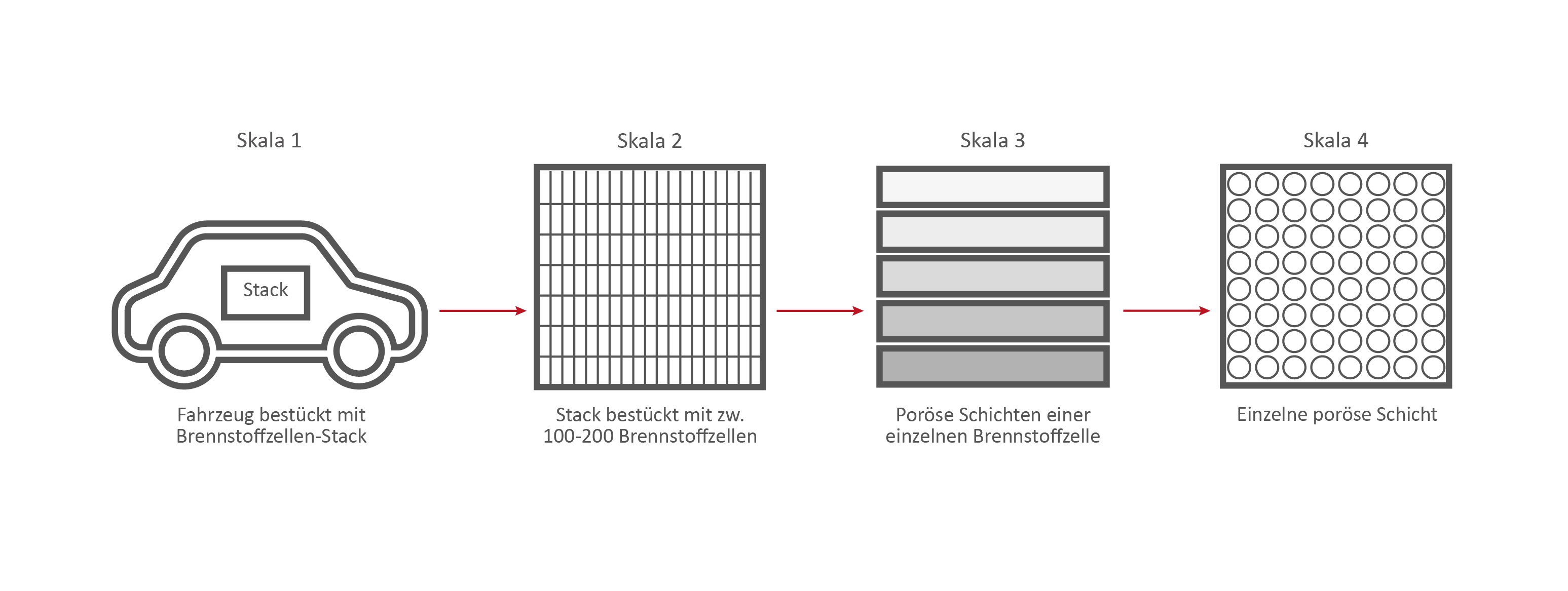
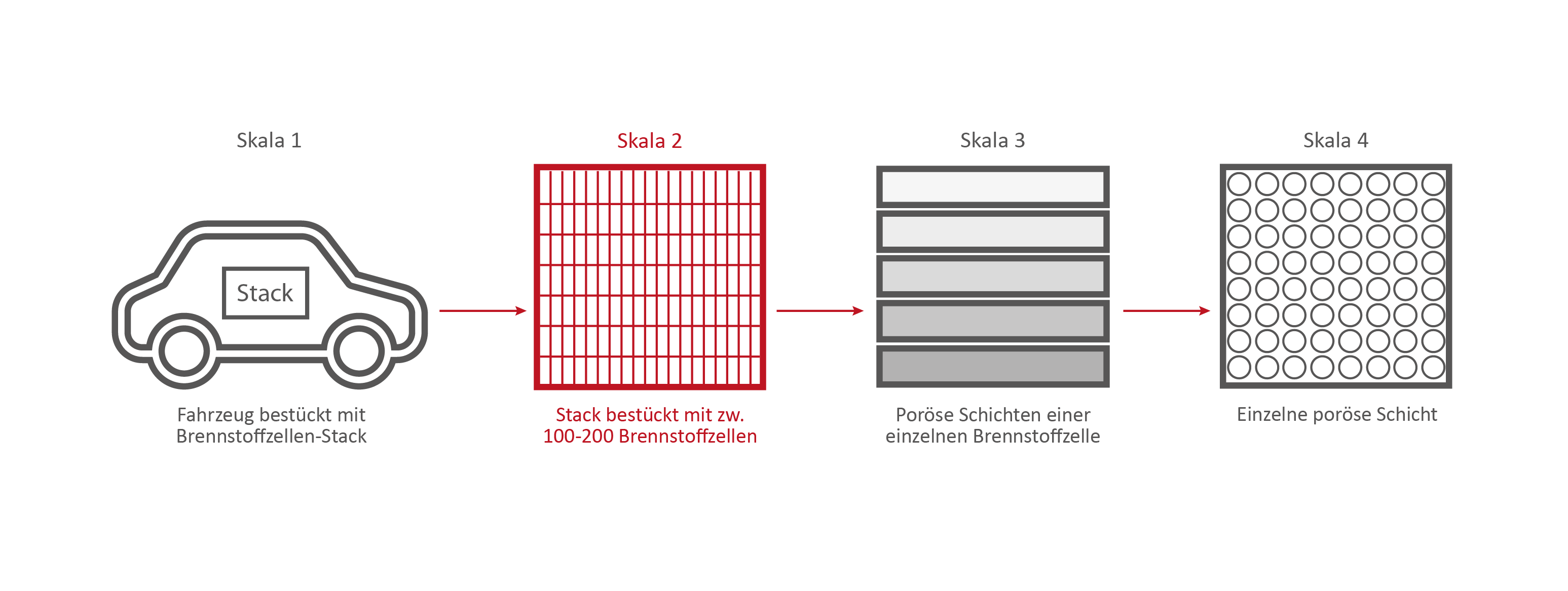
Scale 2: The Fuel Cell Stack
Credits: University of Stuttgart / SFB 1313
Credits: Bosch GmbH
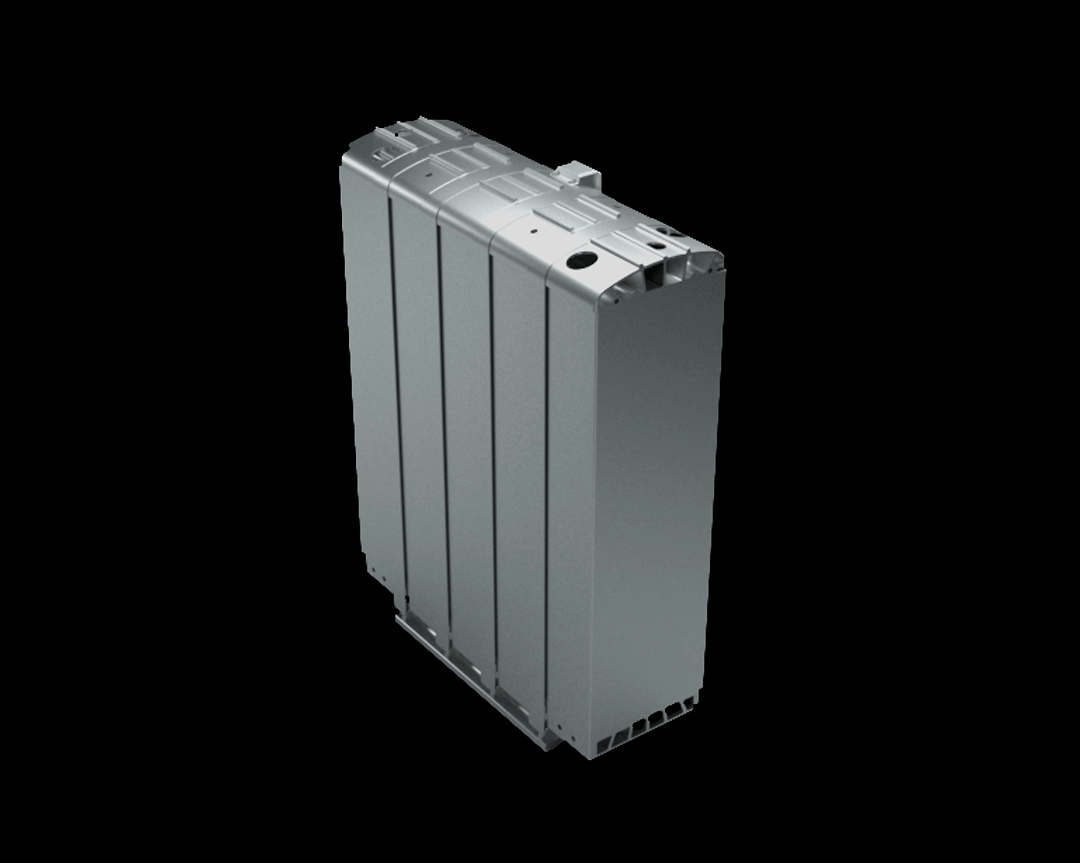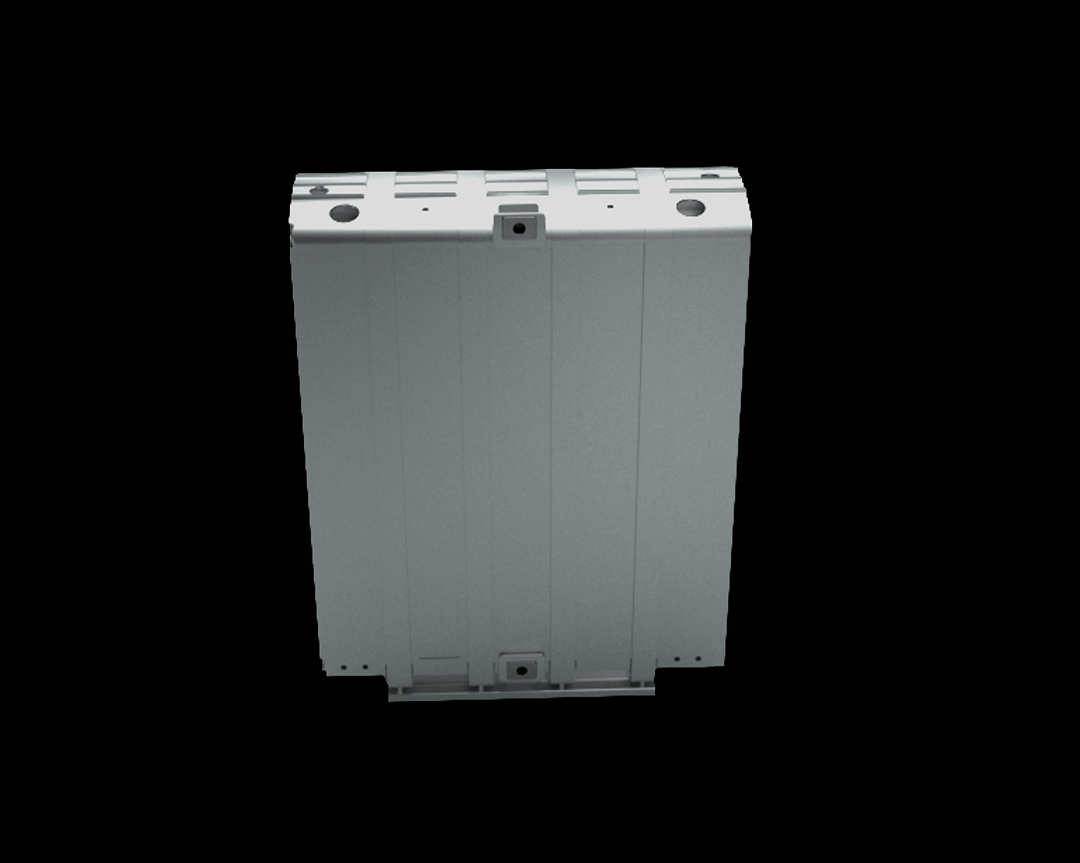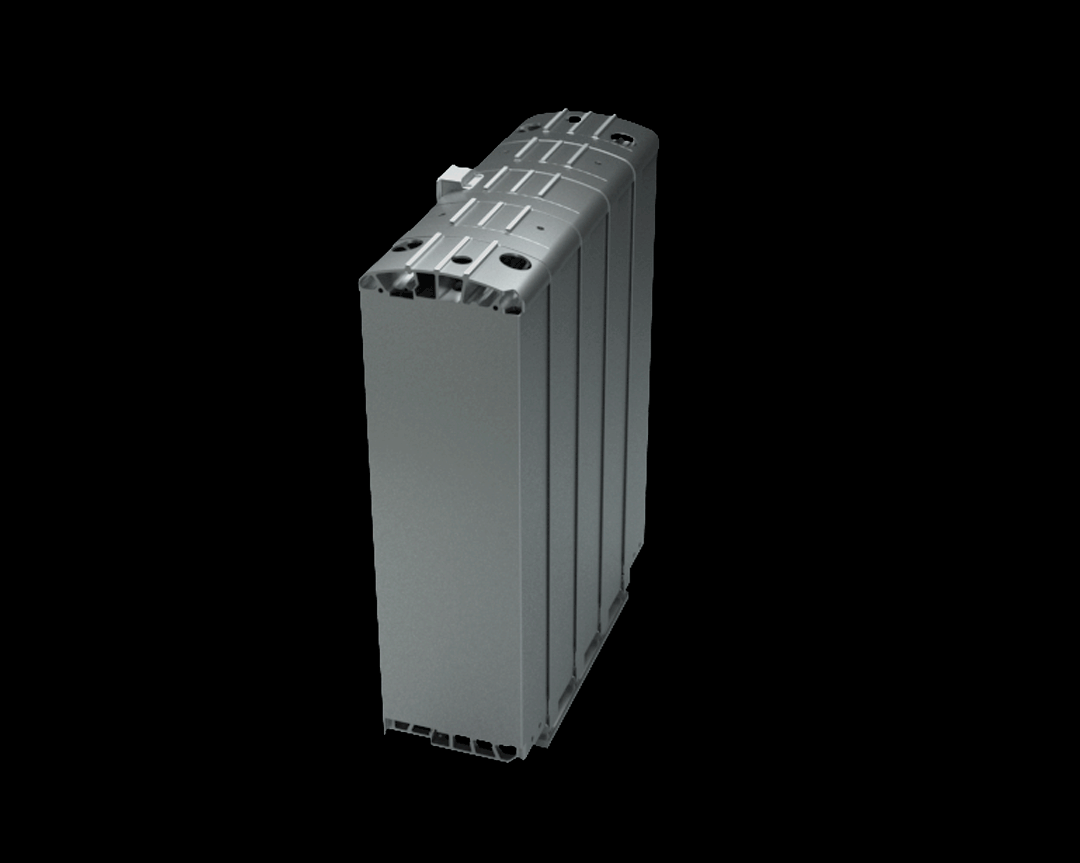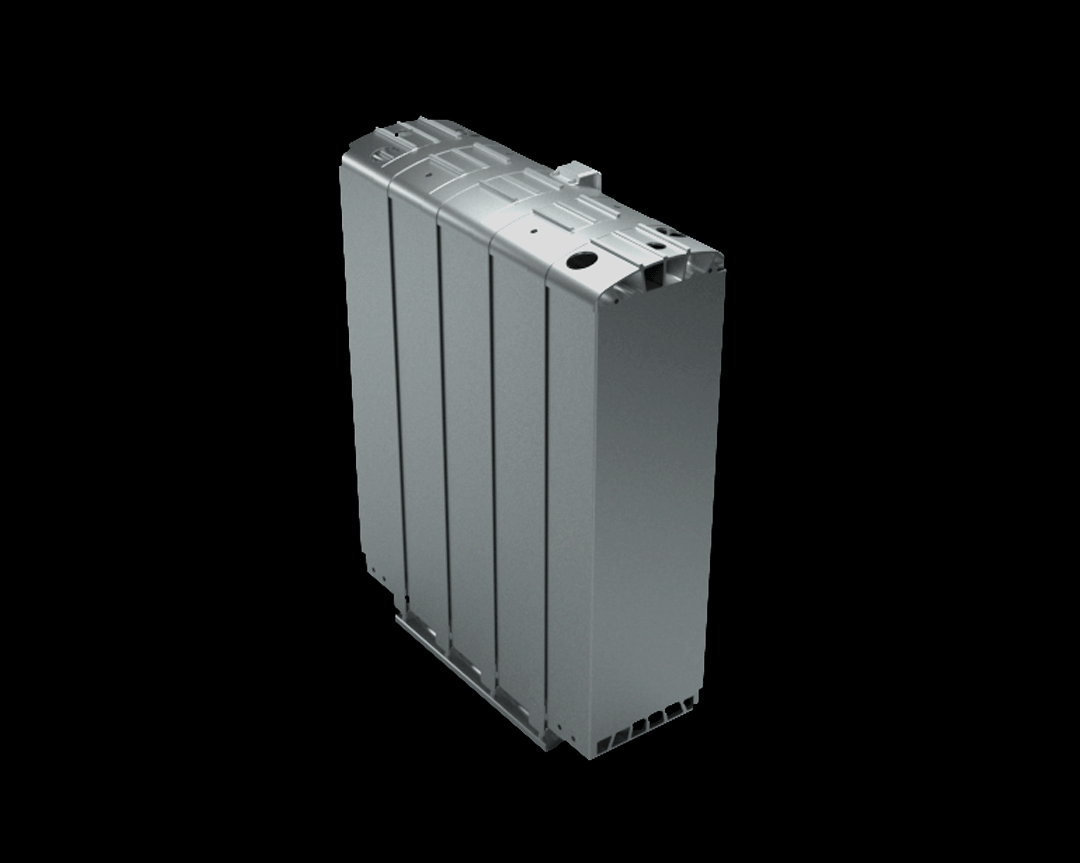
Credits: Bosch GmbH
Credits: University of Stuttgart / SFB 1313
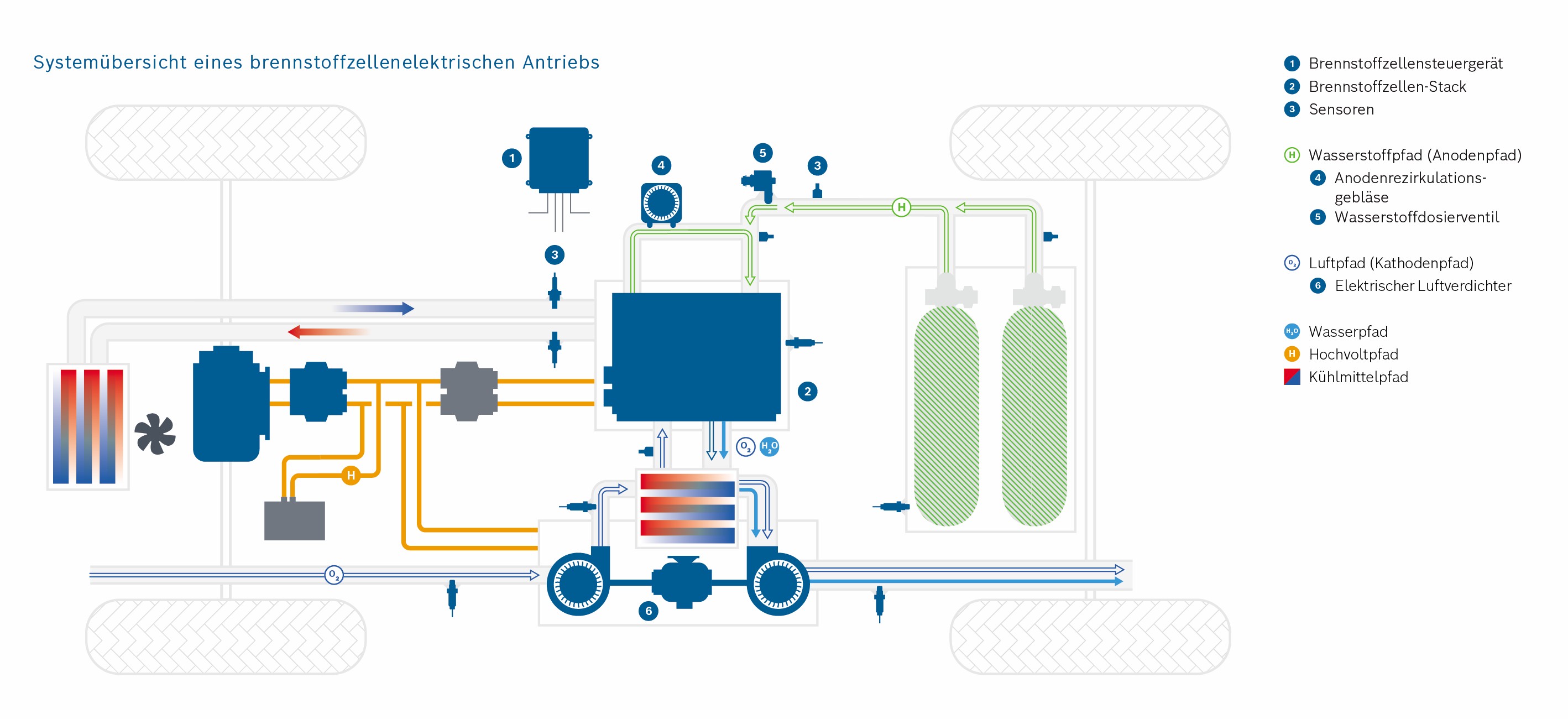
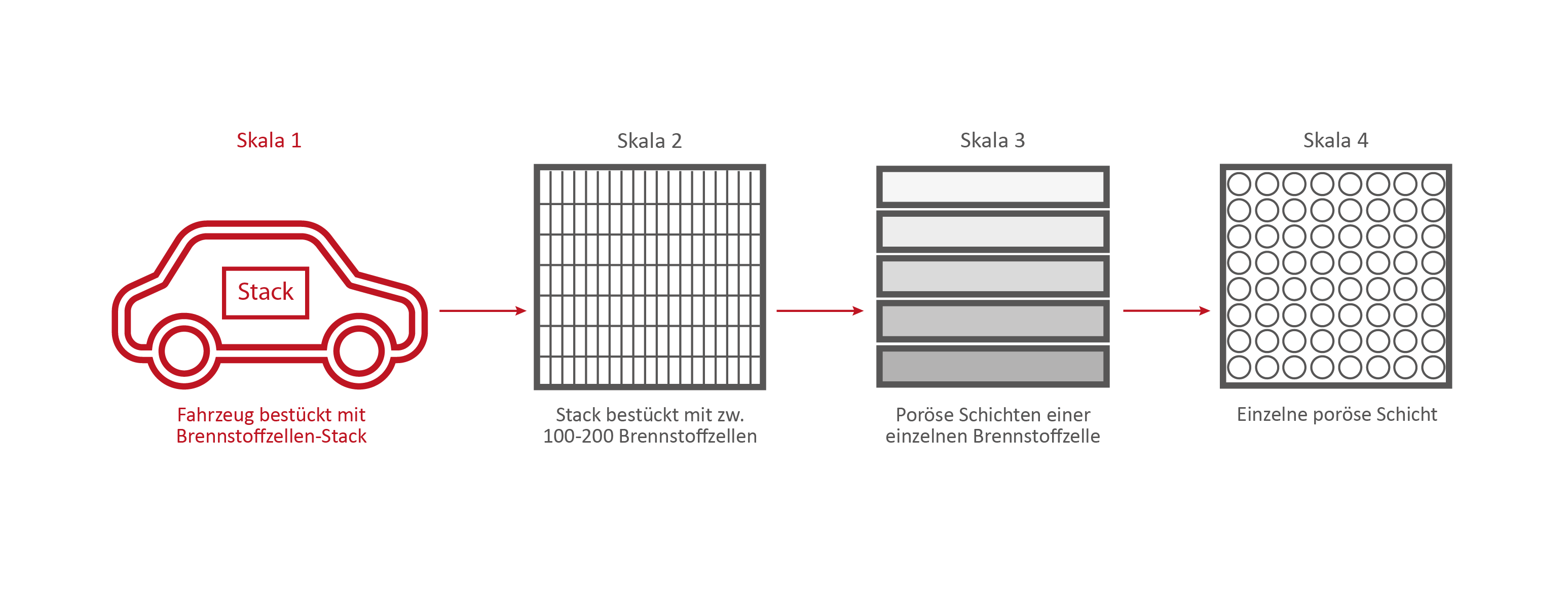
This stack contains several hundred stacked fuel cells. The fuel cell stack generates the electrical energy that the vehicle needs.
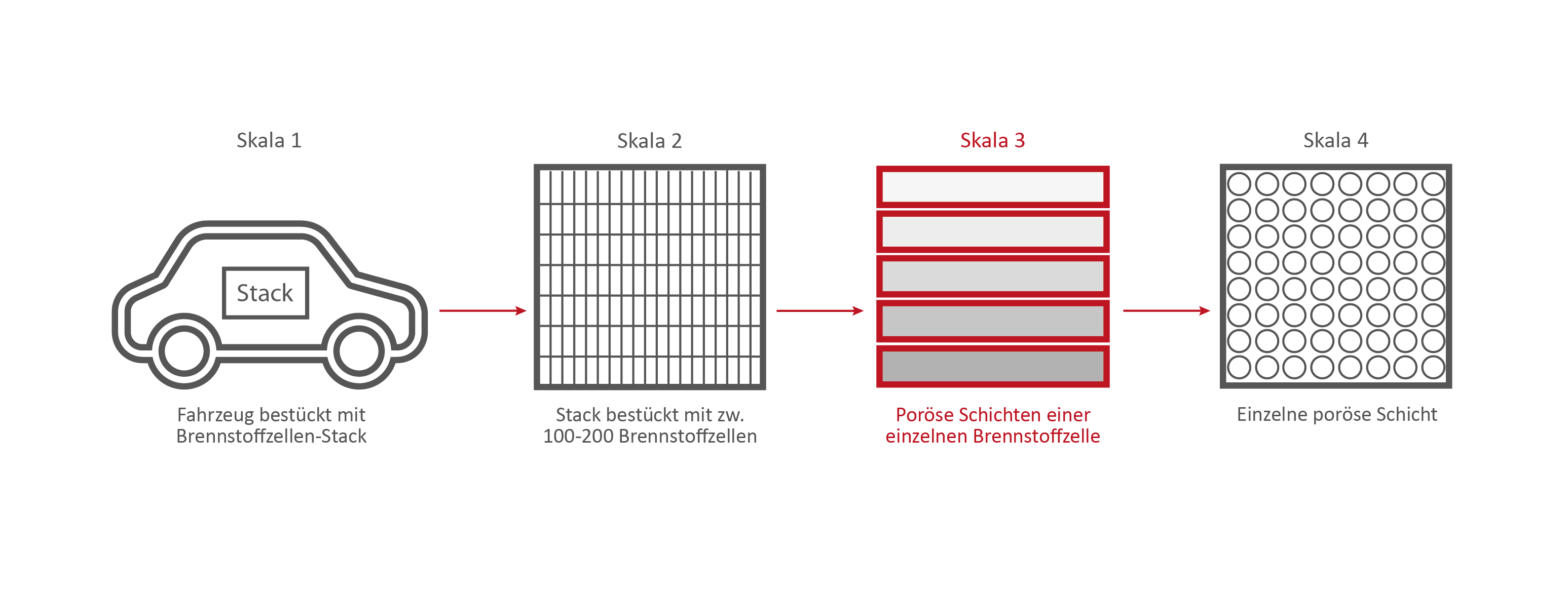
Scale 3: The individual fuel cell and its porous layers
Credits: University of Stuttgart / SFB 1313
The research on the PEM (polymer electrolyte membrane) fuel cell serves as an example. It consists of nine porous and non-porous layers and materials with different properties.
A single fuell cell is very thin, less than 2 cm thick and about 30 cm long.
If the PEM fuel cell is opened, the nine different layers become visible.
Credits: University of Stuttgart / SFB 1313 / Cynthia Michalkowski
Enumeration of the 9 layers of the PEM fuel cell
1) Anode side: Gas channels – Channel structure (not porous). Material: metal or ceramics
2) Anode side: GDL = Gas Diffusion Layer (porous). Material: coated carbon fiber
3) Anode side: MPL = Membrane Polymer Layer (porous). Material: coated carbon particles/grains
4) Anode side: CL = Catalyst Layer (nano-porous). Very fine scale, but the transport processes are the same as in a porous medium. Material: carbon particle and Ionomer chains with platinum as catalyze
5) PEM = Polymer Electrolyte Membrane (more or less porous). The transport is chemical rather than comparable to a porous medium. Material: Nafion
6) Cathode side: CL – see anode side
7) Cathode side: MPL – see anode side
8) Cathode side: GDL – see anode side
9) Cathode side: Gas channel. Classical channel structure (not porous), but can also be expanded metal or similar material that can be interpreted as a porous medium. Material: metal or ceramics.
Hydrogen (H2) and oxygen (O2) (from the air) pass through the porous layers of the fuel cell and finally react to water (H2O) that is transported out of the cell. A “transport” of hydrogen and oxygen through the porous layers of the fuel cell takes place.
Reaction equation of water: 2 H2 + O2 ⇌ 2 H2O
This reaction and the interaction of the various systems in the vehicle generate electrical energy, which ultimately drives the vehicle.
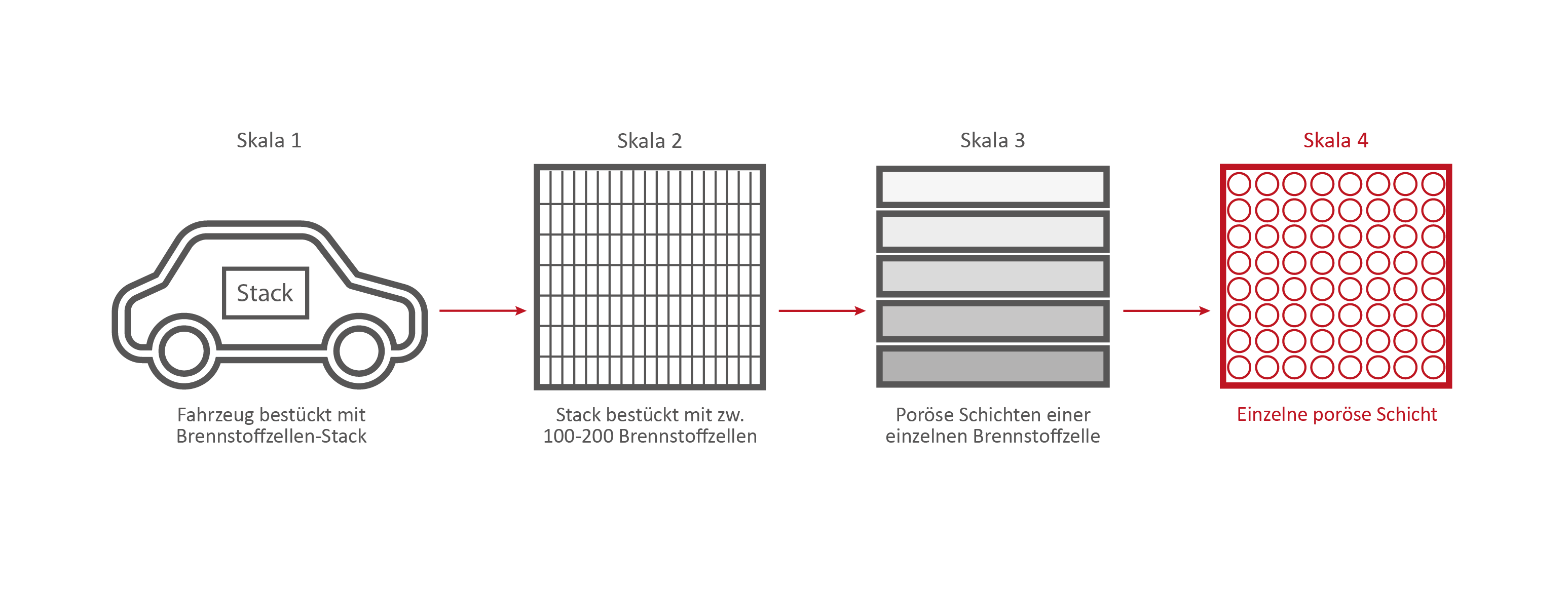
Skala 4: Eine einzelne Brennstoffzellen-Schicht
Credits: University of Stuttgart / SFB 1313
If we take a closer look at one of the nine layers under the microscope – the GDL layer – the network-like structure and cavities become visible. This layer is porous. Hydrogen, oxygen and water can flow through it.
This is the GDL layer, transferred into a computer model. Researchers are able to create simulations with the help of models. This simulation shows how and where exactly hydrogen, oxygen and water flow through the layer.
Credits: University of Stuttgart / SFB 1313 / Cynthia Michalkowski
In the context of mobility change, the fuel cell is becoming more and more important in the automotive world. This technique allows for emission-free driving on a local level. For heavy vehicles in particular, it represents an attractive alternative to battery-powered vehicles.
The PEM (Polymer Electrolyte Membrane) fuel cell consists of multiple porous layers with different characteristics. To remain competitive, high efficiency and durability must be achieved at low cost. Simulations can help us to research the porous layers.
Efficient methods must be developed to describe and model the porous materials, the transport, and interactions of the porous layers. A fuel cell stack, used for example in a vehicle, can be divided into multiple scales (stack scale, full cell scale, representative volume inside the cell, pore scale). The transport processes in the fuel cell takes place on different scales. However, this method is not suitable for the simulation of complete cells or even entire fuel cell stacks due to the computing effort involved. For a precise representation, however, it is important to consider the relevant effects of the small-scale phenomena also on coarser scales. Therefore, intelligent methods must be found. However, to do this, the relevant effects must first be identified. To describe the interaction of the two-phase water-air-transport at the interface between the GDL (gas diffusion layer) and the gas distributor, in this project a pore network model is used. The transport processes are described on the pore scale. A major challenge here is the change of wettability from the hydrophobic GDL to the hydrophilic gas distributor and the resulting occurring phenomena. In addition to modelling on the pore scale, the frequency of the phenomena occurring must also be analyzed. With this information, a stochastically based model can finally be transferred to the full cell scale.
Explanation of the Cathode and Anode
The hydrogen is fed into the cell on the anode side and transported through the GDL and MPL. Hydrogen molecules are split into protons and electrons in the CL, the catalyst layer. This is the anode reaction in the fuel cell. The electrons are transported via the MPL, GDL and the anode gas distributor into the electrical circuit and to the consumer (e.g. electric motor). The protons reach the cathode side of the fuel cell by passing the proton permeable PEM layer. On the cathode side there is a mirrored stack of the porous layers. Oxygen is transported into the cell by the air via the gas distributor and distributed homogeneously on the catalyst layer via the GDL and MPL. There, the oxygen molecules react with the protons which have passed through the membrane into the cathode catalyst layer. During this cathode reaction, electrons are consumed, which enter the cathode from the electrical circuit.
The reaction of protons, electrons and oxygen molecules produces water and heat. Both must be transported out of the cell via the porous layers on the cathode side.
If the water cannot be removed, too many water molecules accumulate in the fuel cell. This leads to a high condensation rate. If there is too much liquid water on the cathode side, the paths through which the oxygen can reach the catalyst layer are blocked. This can lead to a reduction in performance.
If the heat cannot be dissipated, this leads to fuel cell damage. At elevated temperatures, reactions take place in the catalyst layers, which should not take place there and which damage the material. The oxidation processes lead to premature “ageing” of the fuel cell.
Scale 1: The vehicle
Credits: University of Stuttgart / SFB 1313
Credits: Bosch GmbH

Credits: Bosch GmbH
Simulating processes in the fuel cell
Simulations are important in the research of fuel cells because they help to make processes in the fuel cell visible. With their help, researchers can understand the function of the porous layers. They understand how the transport of water, oxygen and hydrogen happens and whether they have to optimize the layers. Should they use a different material or structure? The knowledge they generate from the simulations can, for example, help to reduce costs during the vehicle production process.
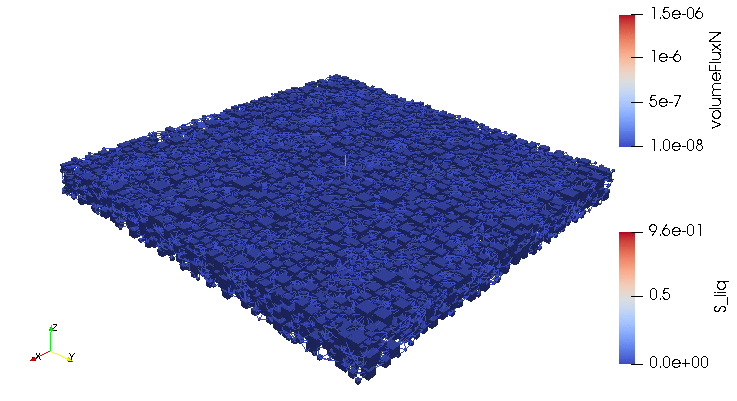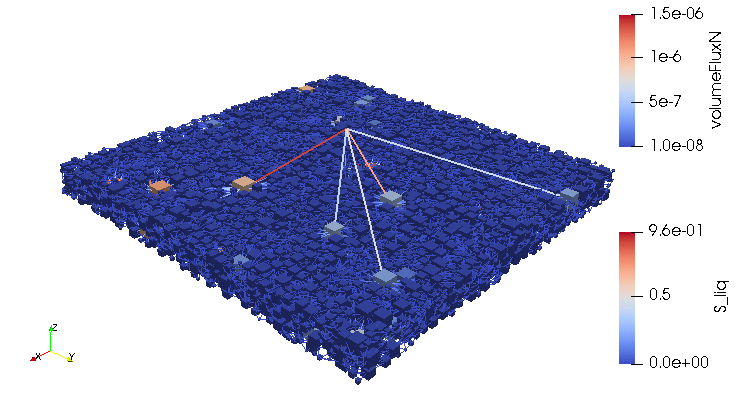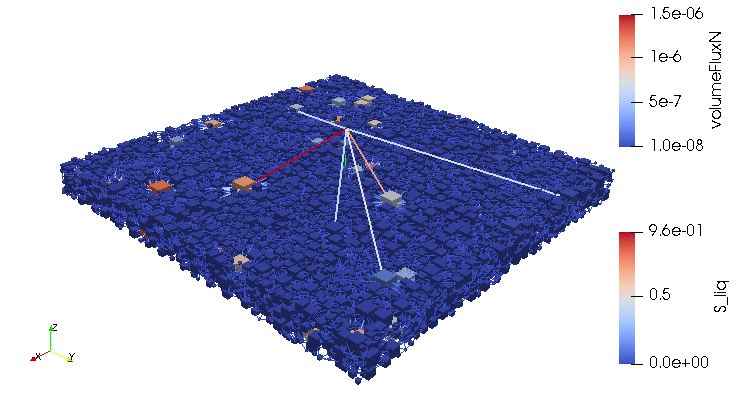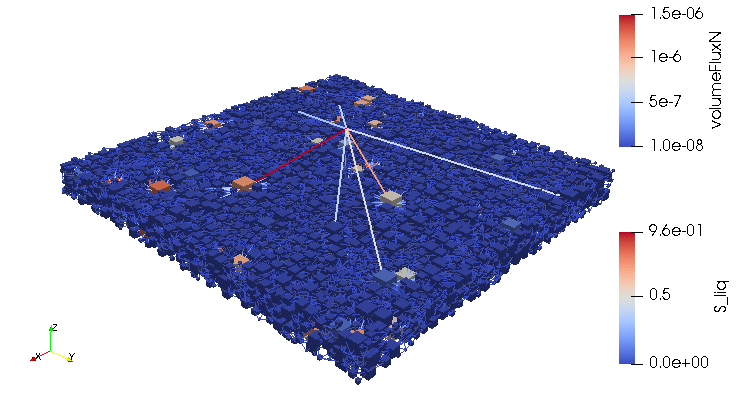
Credits: University of Stuttgart / SFB 1313 / Cynthia Michalkowski
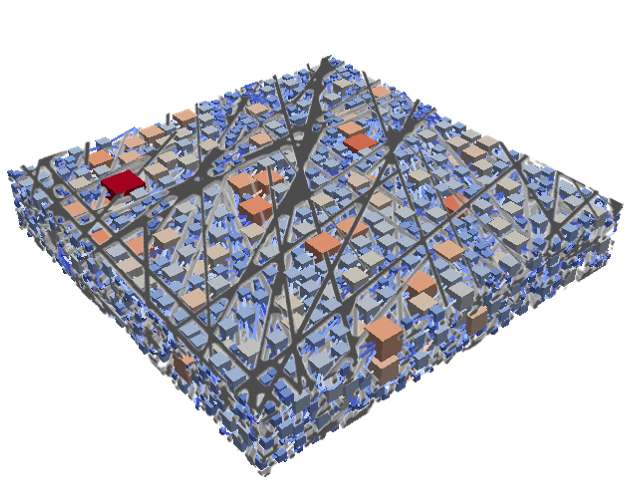
This example shows a model of a fuel cell GDL layer. With the help of this model it is possible to simulate the water proportion in the pores. The water is pressed through the porous layer and exits at the marked points
In-depth text simulation:
During the reaction of hydrogen and oxygen, water is produced. The produced water needs to be removed from the catalytic layer, where the reactions take place, to keep enough space available for new reactants. If there is not no exchange, an equilibrium evolves such that no electric energy is produced by the reactions. To ensure a continuous energy supply, the produced water is removed through the cathode of the cell. The water is transported through the different, porous layers. Water can be present in liquid or vapor form, due to condensation and evaporation. In this simulation, we consider the liquid water transport through the hydrophobic gas diffusion layer. This means, the water is the non-wetting phase on this coated carbon fiber material. On the pore-scale, this is an inhomogeneous and anisotropic structure. To investigate the water transport though this layer and to predict the break-through locations of the liquid water on the surface of the porous layer, a geometrical simplified model is used. The pore space of the material is represented by a network of pore bodies (network nodes) and pore throats (network connections). This allows an efficient calculation of the ongoing transport processes in the gas diffusion layer including the pore-scale effects. The simulation shows the locations, where liquid water first leaves the fibrous material. With this information, the gas diffusion layer structure can be optimized for the desired water distribution.
Pretty Porous
06
06
Technology
Future mobility options – using the example of the fuel cell
We also find porous media in the field of “technology”. The materials used for machines and their accessories are very often porous. This is also the case in vehicles.
Credits: Natalie Weinman, Sven Tillack, Steffen Knöll
How to power vehicles?
1) Engine: Vehicles powered by a combustion engine emit climate-changing CO2 and environmentally harmful pollutants into the atmosphere.
2) Battery: Battery-powered vehicles are powered by electrical energy. The so called electric drive. Either the energy is stored in a battery, which has to be recharged, or via power grids, which permanently supply the required electricity from outside. This is, for example, often the case in public transportation.
3) Hybrid: In the hybrid vehicle, the electric drive is combined with an additional drive, for example via a motor.
4) Fuel cell: Fuel cell vehicles are powered by hydrogen.
In this case, we show porous media using the example of the fuel cell, which is used, among other things, to power vehicles. Hydrogen and oxygen flow in channels through the fuel cell and are transported through various permeable layers to ensure a controlled reaction of the components. The reaction in the fuel cell generates electrical energy, heat and water.
Explanation of the Cathode and Anode